Berry Consultants announces a significant upgrade to FACTS (Fixed and Adaptive Clinical Trials Simulator). Users can now design and compare 3+3, BOIN, mTPI-2, i3+3 and CRM (using BLRM). This makes FACTS the most comprehensive suite of dose escalation designs on the market. The CRM includes a rich set of additional features such as back-filling, open enrolment, and simultaneous assessment of efficacy and toxicity.
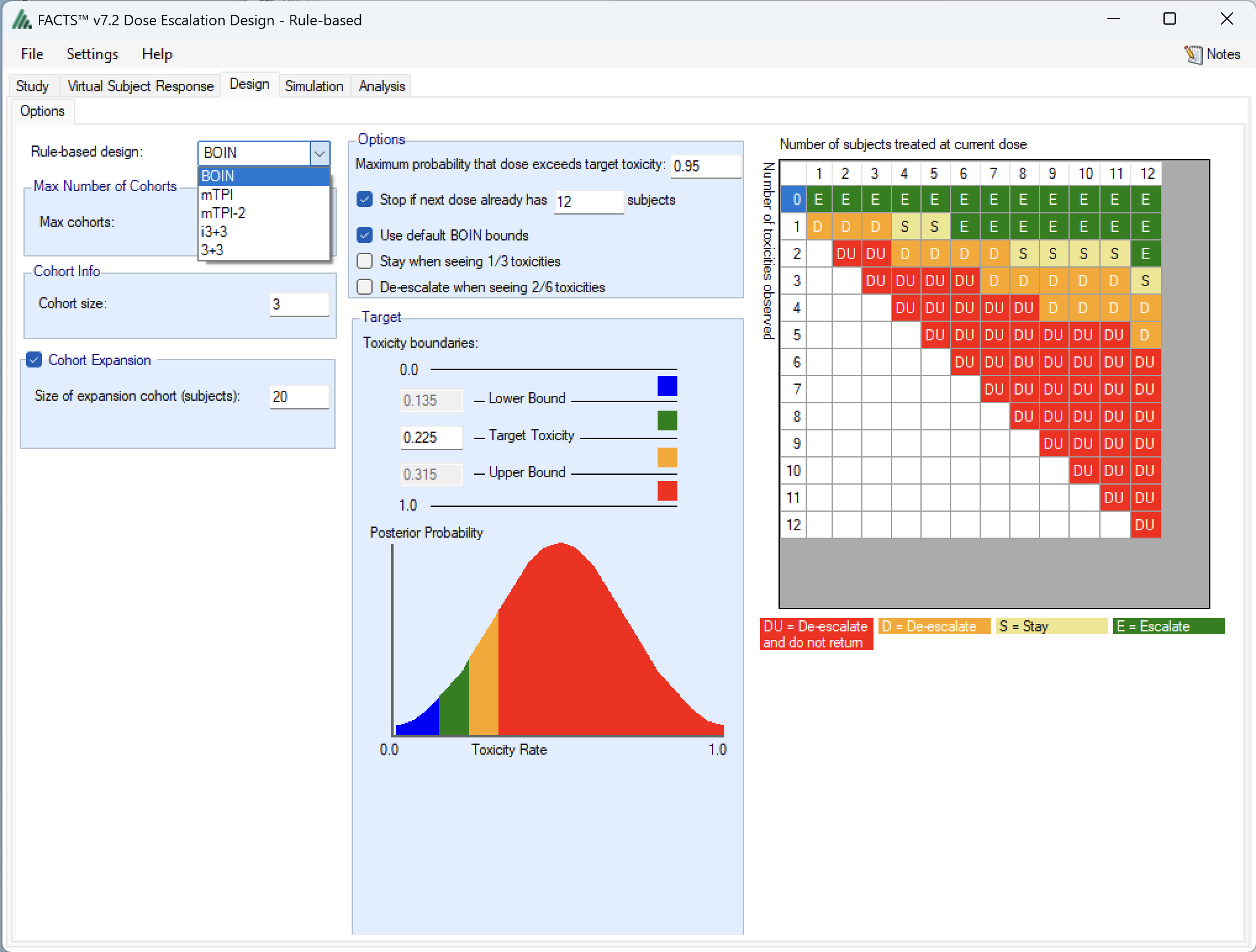
The advantage of having all these methods in one place is simple – it makes it particularly easy to compare and contrast the designs. Having created a design using BOIN for example, it is as simple as changing the selection on the “Design > Options” tab from “BOIN” to, say “i3+3” and saving the design to a new file.
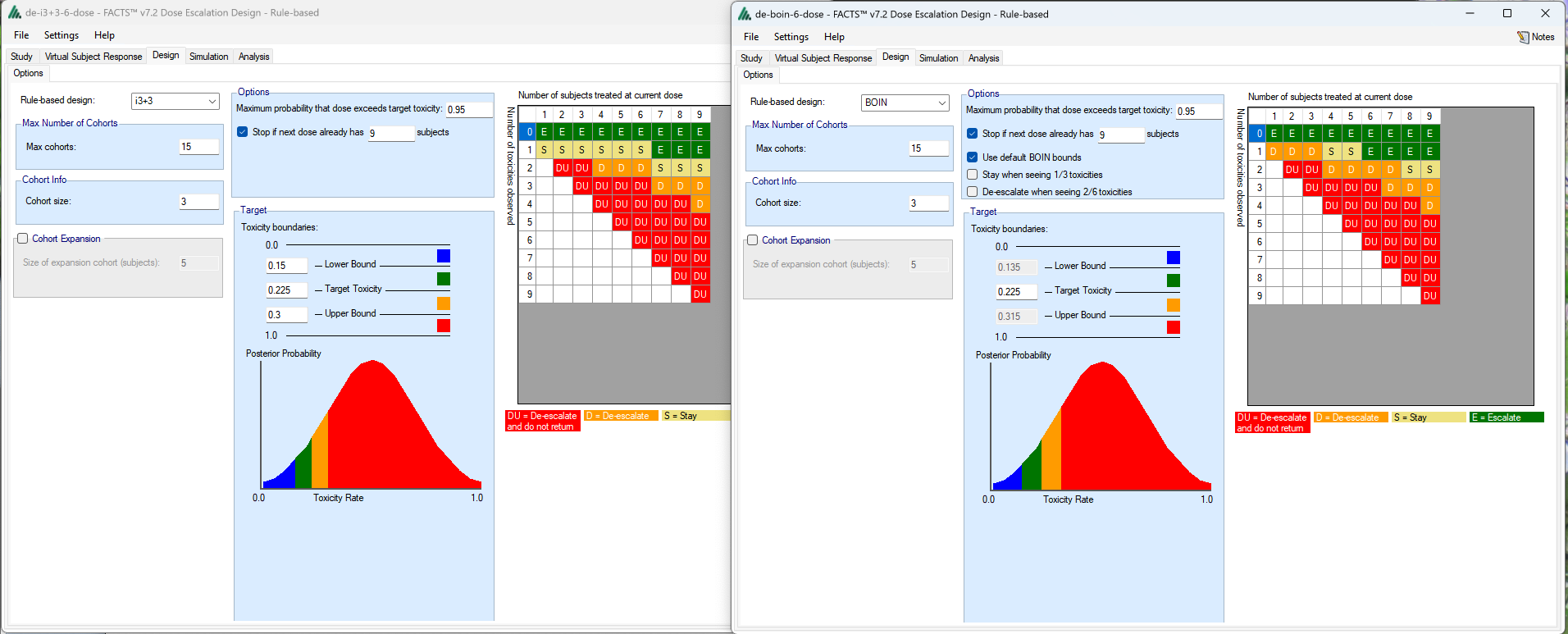
We can see how the decision tables differ:
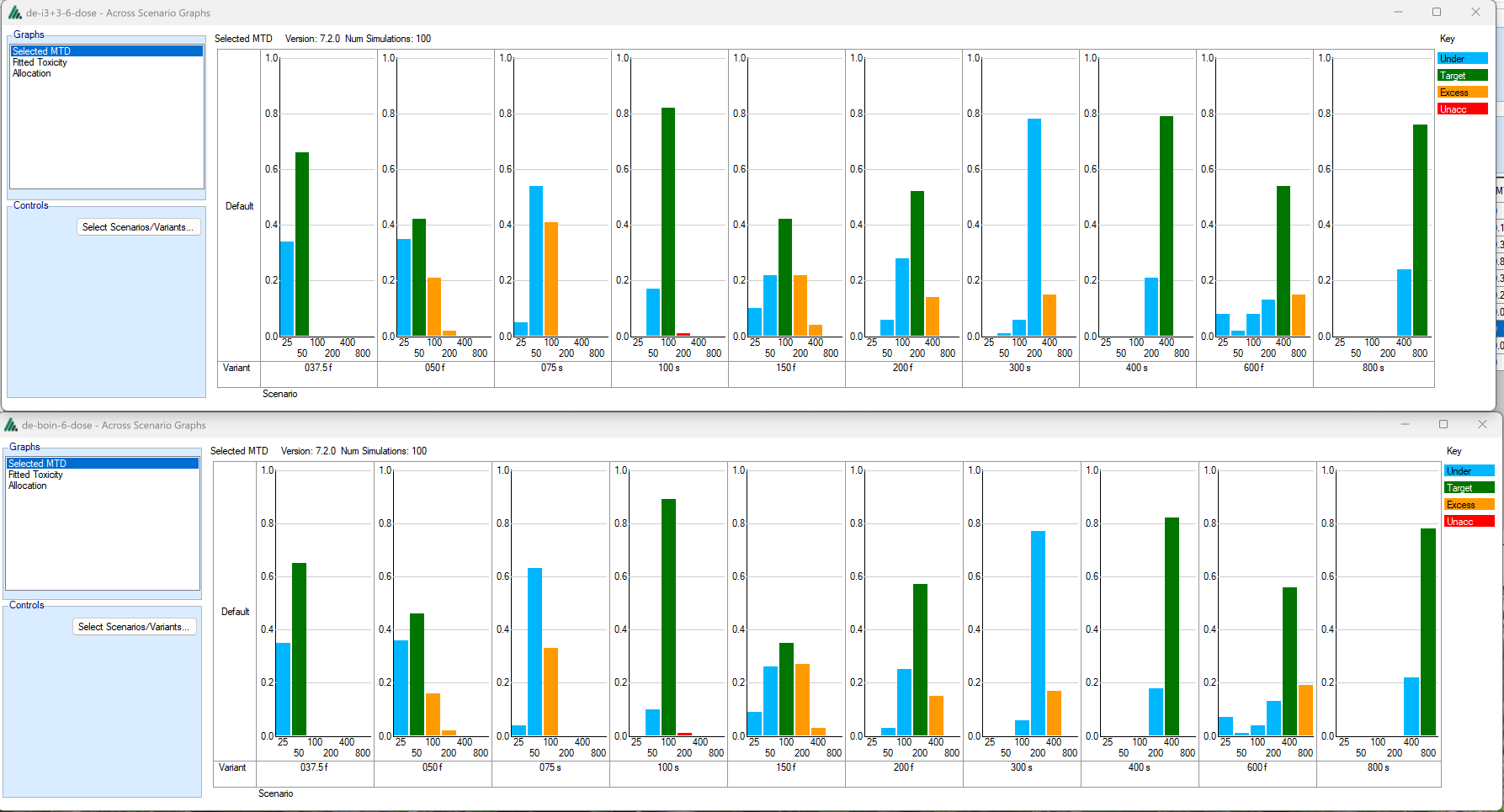
Compare the (here very similar) operating characteristics in which dose they select as MTD across a variety of scenarios:
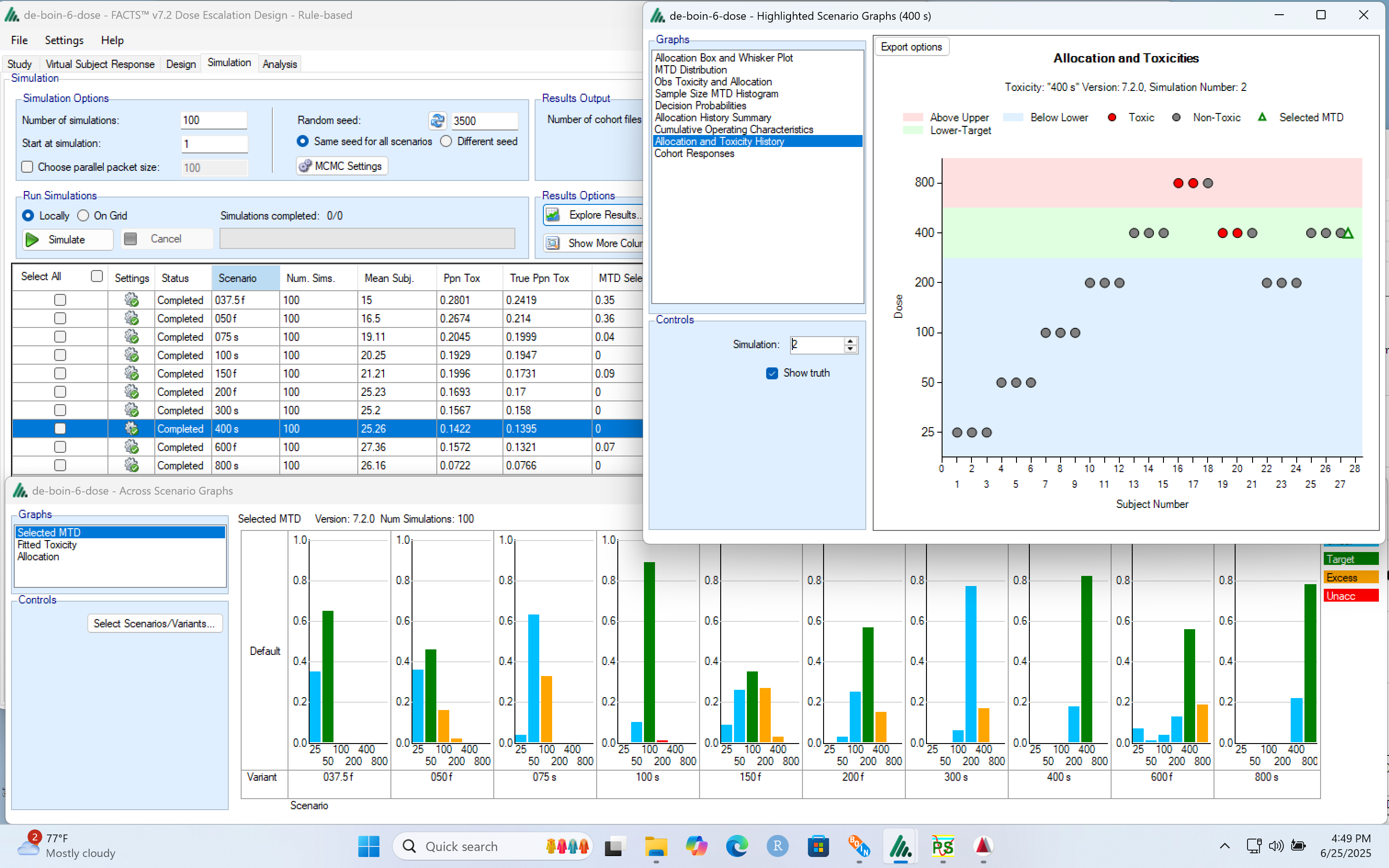
And look at how they perform at the individual trial level:
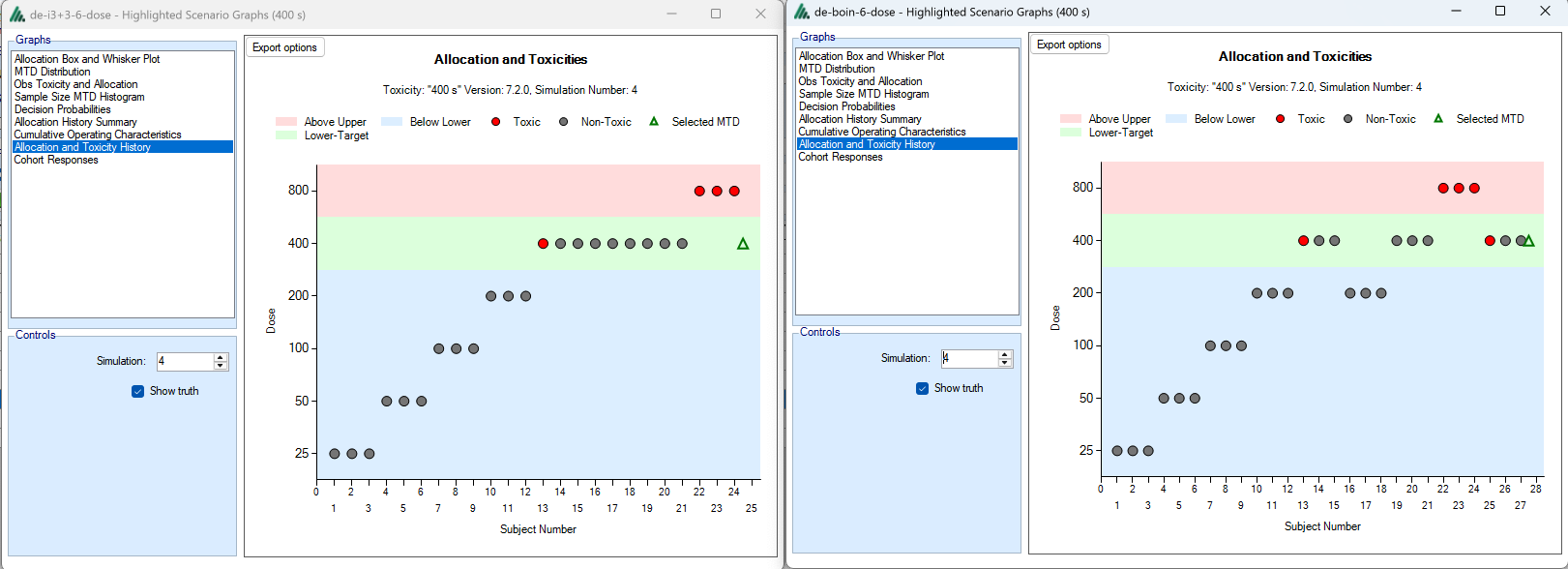
A crucial attribute of FACTS is the recording of every simulation, both allowing the properties of a design to be reviewed and communicated in detail, and also the post processing of simulations for deep analysis and exploring “what-if” questions.
This update reinforces FACTS as the leading integrated tool for fast, efficient, accurate, and innovative clinical trial design and simulation.
For further information, please contact us.