April’s FACTS webinar focused on Longitudinal Models in FACTS. It’s a topic that we could spend 8 hours straight on and not get a full review done, but we squeezed a lot into the hour. The slides are attached here.
The general structure of the webinar focused around an example trial similar to the lecanemab phase II trial. Scott Berry presented that trial as a case study in a 2024 FACTS webinar, complete with about a 3 minute clip from the movie Amadeus woven in. We left out some of the adaptations used in that trial, and focused on the longitudinal aspect of the ADCOMS endpoint across 3-, 6-, 9-, and 12-month visits.
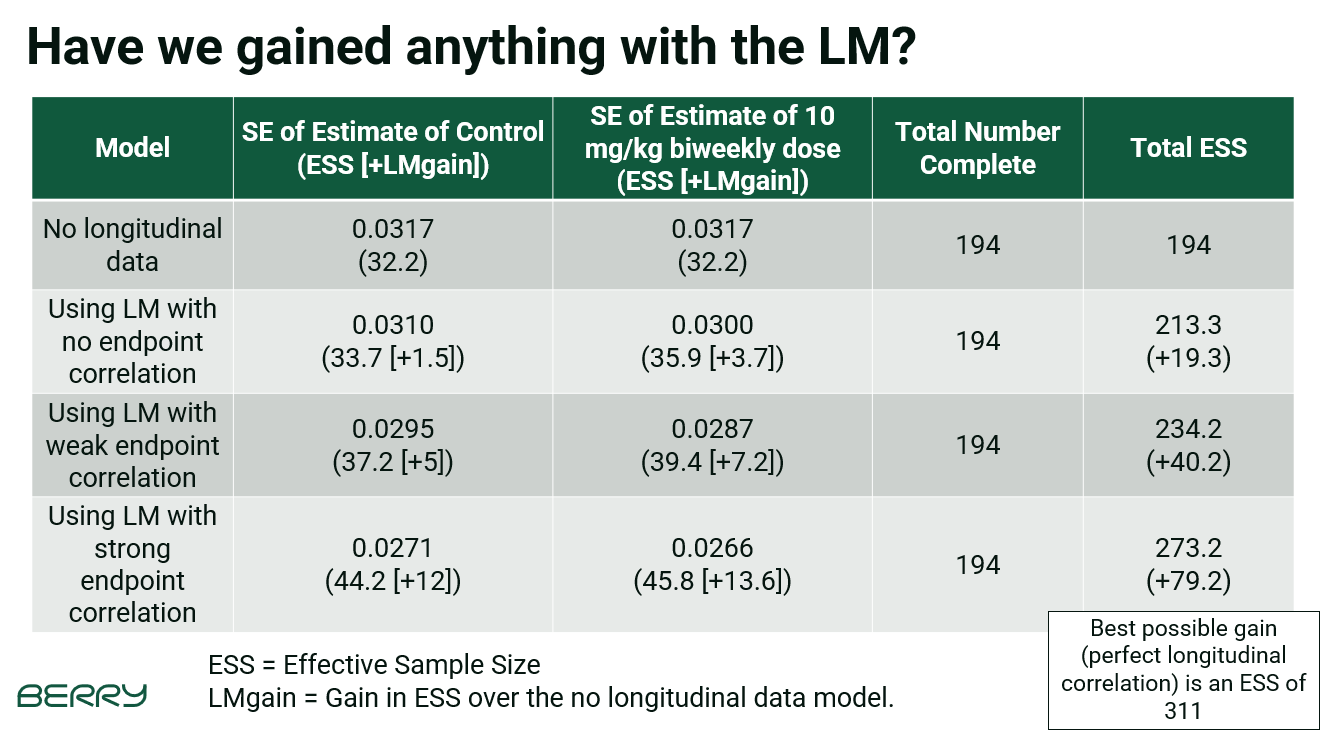
We first assessed the efficiency gains in the estimation of the mean response for the doses in the trial at the 4th out of the 13 interim analyses. At that point there is roughly 194 complete subjects and another 117 with intermediate, but not complete, data. The longitudinal model increased the effective sample size at the analysis (based on the precision of the posterior distribution) by up to 80 subjects. This efficiency gain of course depends on the assumed correlation of the intermediate data with the final endpoint data.
Then, we moved past the interim estimation precision to operating characteristic benefits. We aimed to equalize the power across the trials with and without longitudinal models and with varying intra-subject correlation. The improvements in the trial, then were expressed in terms of average sample size. When the correlation between intermediate and final endpoints was large, the average sample size of the trial could be reduced by up to 13% in certain scenarios. In our simulated trial the longitudinal model was especially good at speeding up early stopping for success decisions.
The FACTS files used to make OC comparisons are: