Study Info
The Study Info sub-tab provides parameters for specifying rules and methods for common clinical trial simulation features. These include accrual style, visit schedule, whether interim analyses will be simulated, and more.
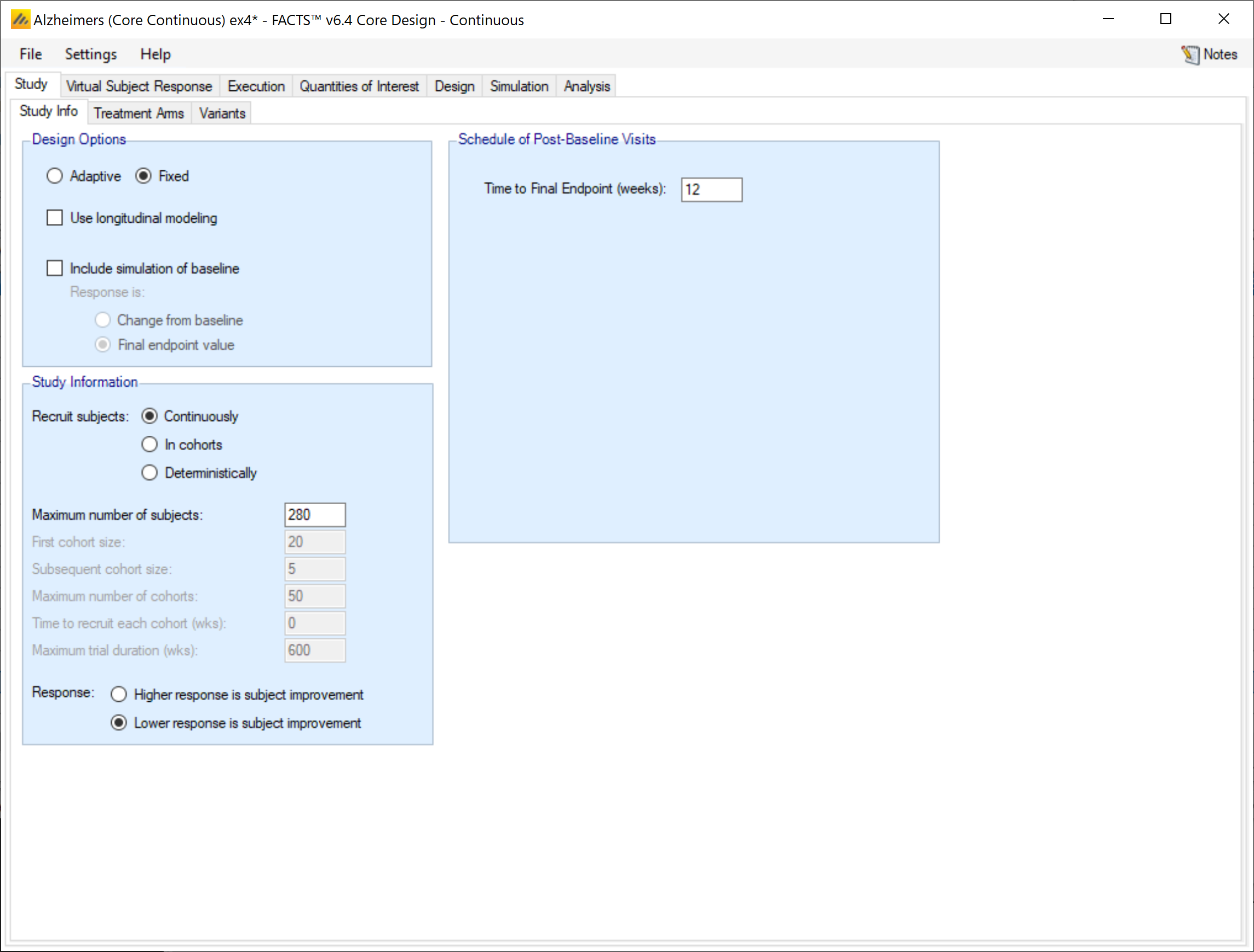
Design Options:
In the design options section of the Study tab the user gets check boxes for whether they want to enable adaptive features, use longitudinal modelling, or include simulation of baseline. These options have the following effects on the trial simulation.
Enable adaptive features
Whether the design is adaptive or fixed. If “adaptive features” are enabled, some adaptive specific parameters and tabs are added to the GUI, such as the tabs for defining interims, early stopping criteria, and adaptive allocation.
Use longitudinal modeling
Whether longitudinal modeling is going to be used. If longitudinal modeling is not selected, some longitudinal specific parameters and tabs are hidden. If use longitudinal modeling is selected, FACTS expects that the early endpoint data will be used, it cannot be ignored. If intermediate data is not intended to be used in the modeling, then it should not be simulated in FACTS.
Include simulation of baseline
Whether to include simulation of subject’s baseline score, and if so, whether the response to be modeled is change from baseline or final endpoint value.
Study Information:
The study information section allows the user to specify how subject accrual should be simulated, and whether a larger endpoint value indicates improvement or not.
Recruit Subjects
Subject accrual can be configured to be done continuousl, in cohorts, or deterministically.
If recruited continuously, the user recruitment will be simulated stochastically with a Poisson process, using the parameters specified on the Execution > Accrual tab.
If recruited in cohorts the user specifies:
The size of the first cohort
The size of subsequent cohorts
The maximum number of cohorts
The time (in weeks) to recruit each cohort
The “Maximum number of subjects” and “Maximum trial duration” fields are automatically updated to reflect the cohort parameters entered.
If recruited deterministically, the user specifies the recruitment date of every subject recruited by uploading a file of dates on the Execution > Accrual tab.
Response:
Indicate whether a larger endpoint value indicates subjects improvement or if a lower endpoint value is better. The value provided here informs the direction of frequentist hypothesis tests All FACTS frequentist tests are 1-sided. and with the evaluation of QOIs like Pr(Max) and predictive probabilities.
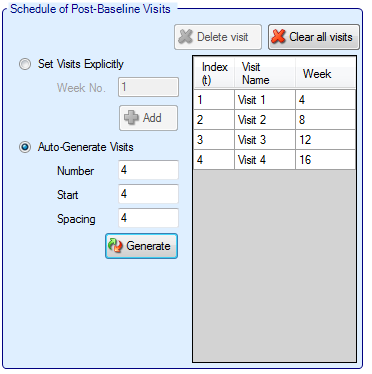
Schedule of Post-Baseline Visits
If “Use longitudinal modeling” is not checked, then the post baseline visit specification is simple. The only required entry is the time it takes to observe a subject’s final endpoint.
If “Use longitudinal modeling” is checked, the follow-up period for subjects is slightly more involved. Visits are specified one at a time, by entering the required week value for the visit and then clicking ‘Add’, or by specifying a regularly spaced sequence: select ‘Auto-Generate’, enter the number of visits, the week of the first visit and the number of weeks between each visit and then click ‘Generate’.
Individual visits can be deleted by selecting them in the list and clicking ‘Delete’. The default visit names can be edited by clicking the visit name and typing. The week and the index cannot be changed. Should it be necessary to change the week of a visit, the incorrect visit must be deleted and a new one with the correct week number added.
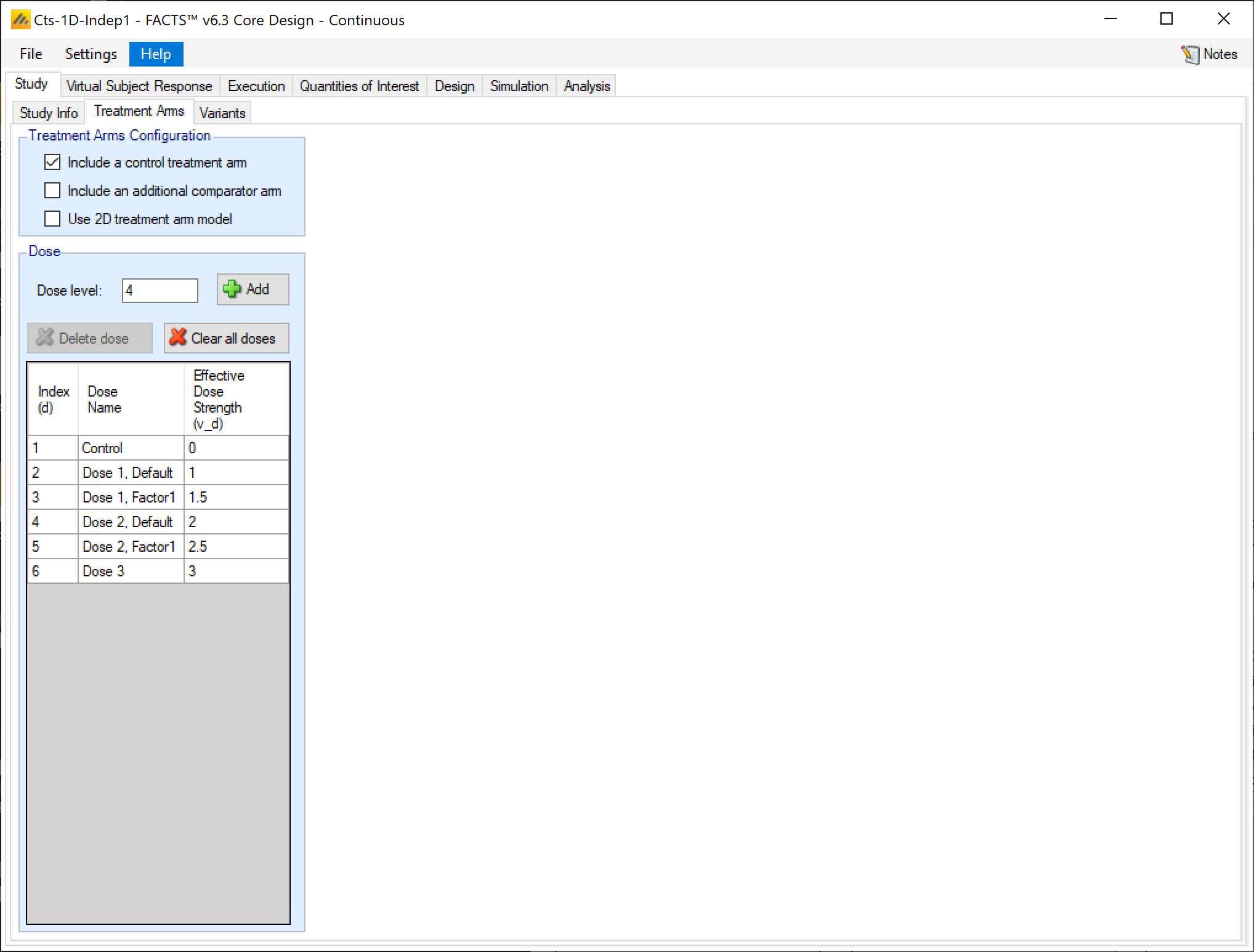
Treatment Arms
As with all FACTS Core engines, the Treatment Arms sub-tab provides an interface for specifying the various dose levels, Control and Additional Comparator arms, and whether using a 2D treatment arm model.
The user may add doses either explicitly or by auto-generation.
The index column provides the ordering of the doses, as well as the number that will be used to subset the dose in dose response models and FACTS output. The dose name column is editable by the user, and is used to identify the doses in the VSR tab as well as in the simulation output shown on the Simulation tab. Effective Dose Strength (\(v_d\)) are the relative strength values of the doses, and are the values used in the dose response model analysis as \(v_d\). They have no units, but the proximity of doses to other doses will determine them amount of information sharing that occurs between doses in certain dose response models. Dose levels may not be edited directly The doses in the design become linked to many other parameters, if dose levels could be edited this could change the effective order of doses and keeping the other parameters settings associated with the right doses becomes problematic – in different circumstances the user might want values to stay with the particular treatment arm or stay with the particular slot in the treatment arm ranking. Forcing doses to be deleted and re-entered if their dose level changes – to change a dose level, delete the entry and add a new dose with the correct level.
If “Include a control arm” is selected at the top of the Treatment Arms tab, then a control will be included in the table. Including a control arm causes many default QOIs to compare to control and sets frequentist tests, predictive probabilities, and conditional power to compare against the control arm rather than an OPC, among other things.
The active comparator arm can be compared against in posterior probability QOIs, and is always modelled independently in the dose response model.
2D Treatment Arm Model
If the user checks the “Use 2D treatment arm model” option, the screen changes to allow the treatment strengths to be specified on 2 axes, and to specify which of the treatment combinations thus created will actually included as arms in the trial.
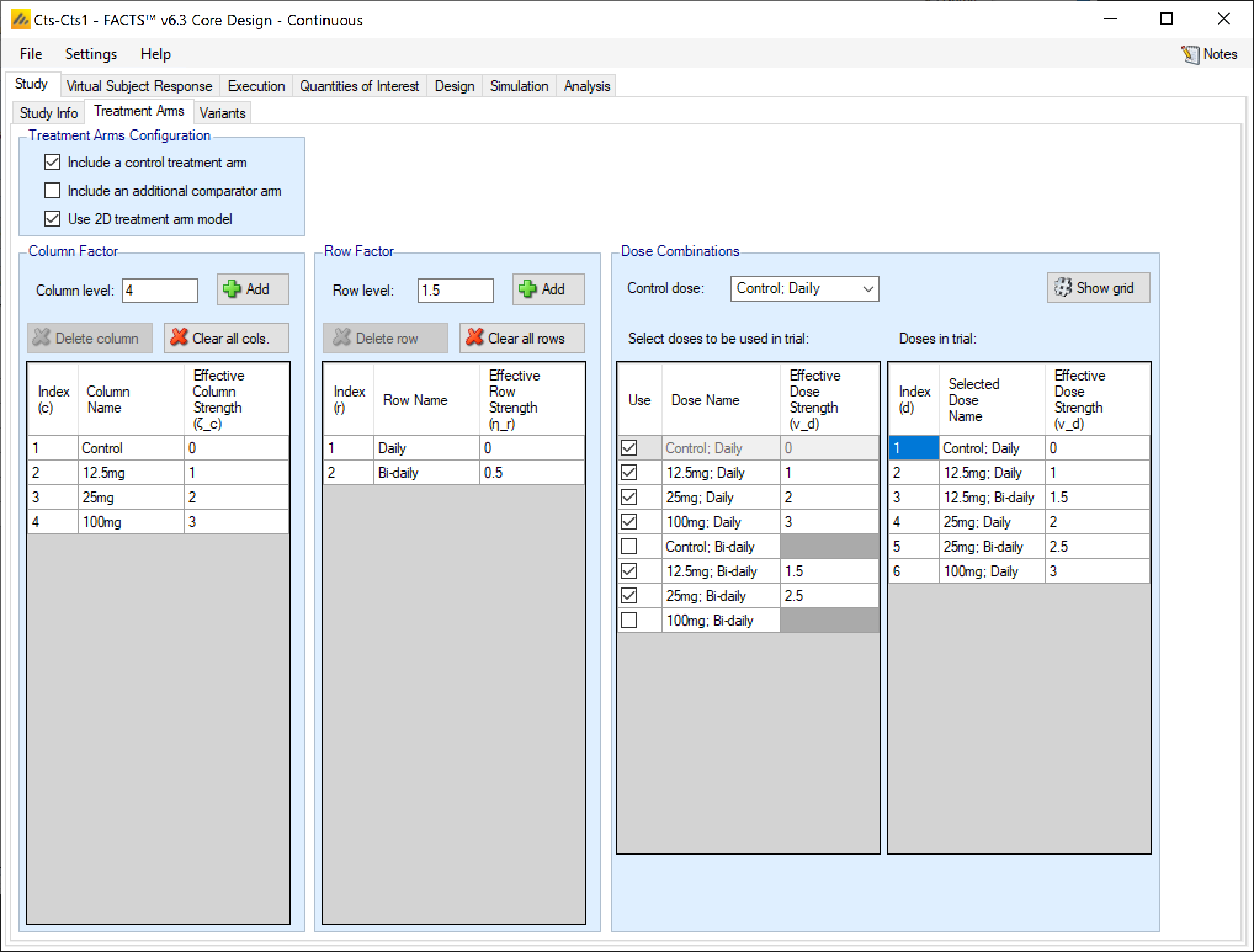
The user now specifies strengths as “Column Factors” and “Row factors”. For example, the Column factors could be different dose strengths and the row factors could be different dosing regimens, or different treatments that the investigational treatment could be combined with. Because of the way the factors are shown in FACTS graphs it is better to use the factor with the greater number of values as the column factor. From the point of view of the analysis however the factors are treated symmetrically. In FACTS we have adopted the neutral term “factor” to avoid the impression that these designs can only apply to doses and dosing regimens[^3].
FACTS then shows all the potential combinations of column and row factors in the list “Select doses to be used in trial” in which the user selects which of the factor combinations that will be present as arms in the trial. FACTS constructs the “effective strengths” opf the combinations by simply adding together the strengths of the two factors, but while the strengths of the factors (like the strengths of “doses” in the 1D setting) cannot be simply changed (because of the problems of managing the ordering of the factors), strengths of combinations can be directly edited, and indeed must be edited to resolve any “ties”. All combinations must have unique strengths so that they can be ranked in order.
If a control treatment arm is included in the trial then a “Control” factor is included in the Column factors and the user selects which combination of this factor and a Row factor is the Control combination. This combination is given the effective strength 0 and moved to the head of the list of doses. If the “include a control treatment arm” option is checked, there must be a combination that acts as control dose. If the option is not checked there will be no control dose.
FACTS displays the resulting ordered list of combinations in the “Doses in trial” column.
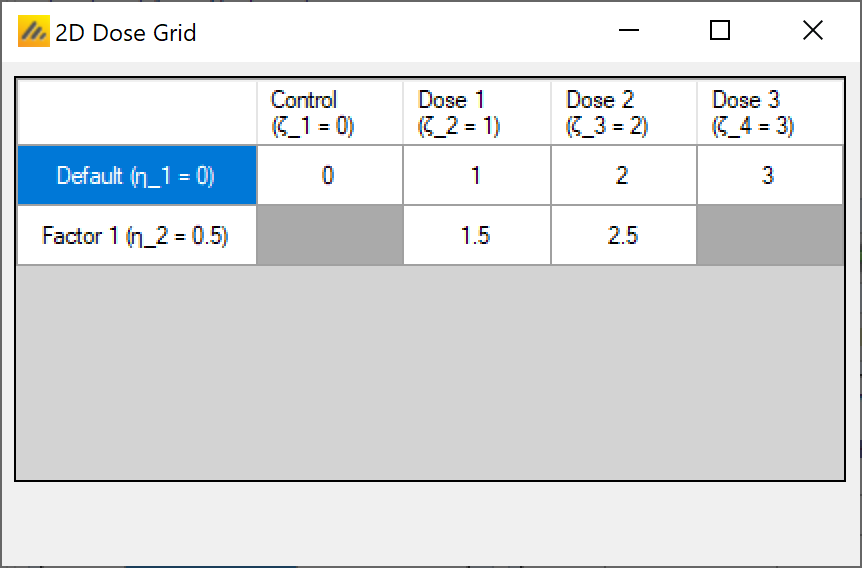
There is also a “Show grid” option that will display a small graphic of the 2D layout of these “Doses in trial”:
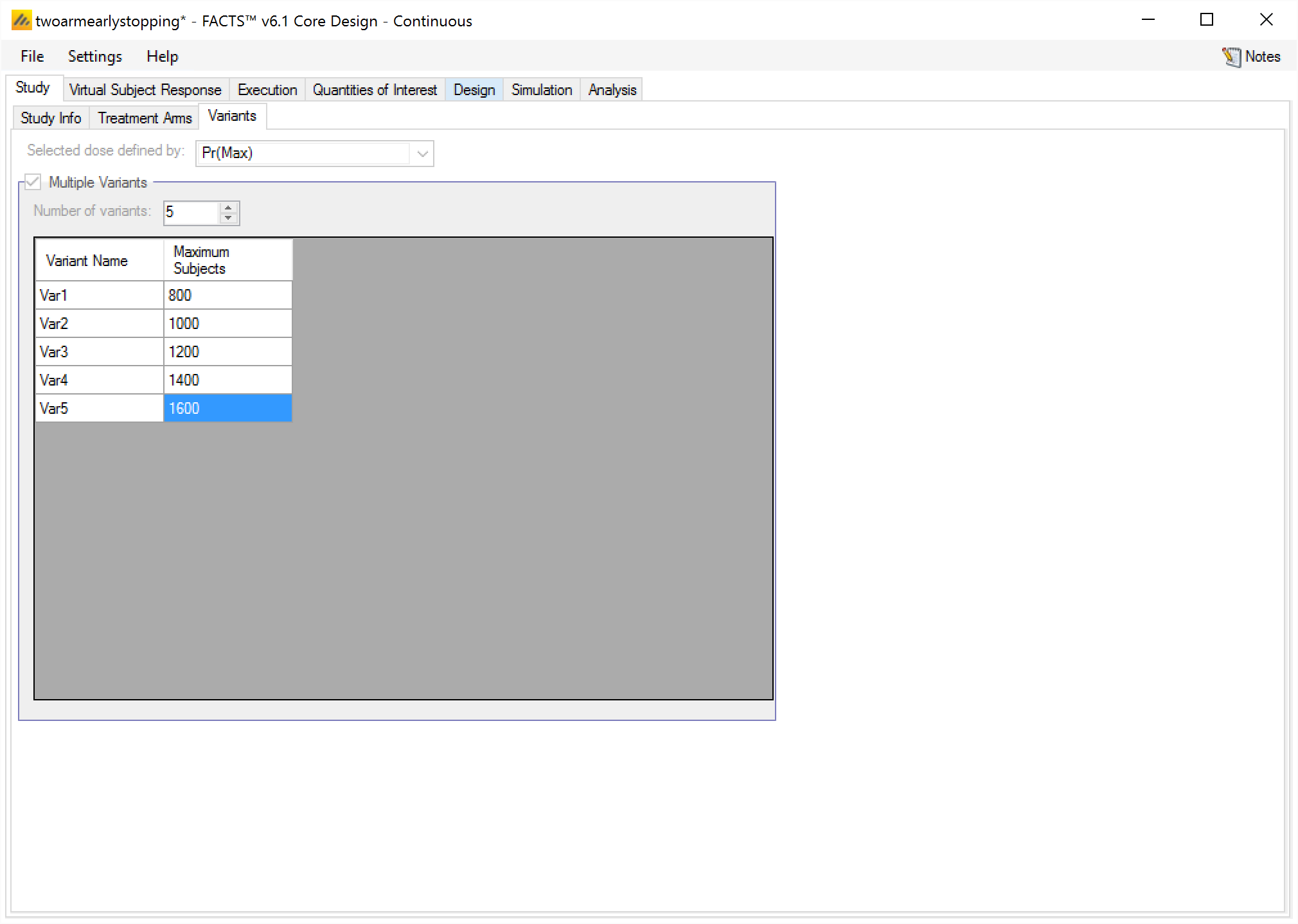
Variants
On this tab the user can specify that a number of design variants should be created. Currently, the only design feature that can be changed is the sample size (maximum number of subjects).
If “multiple variants” is checked, then the user can specify that simulations setups should be created for each simulation scenario with versions of the design with a different maximum number of subjects.
The user enters the number of variants they wish to create. Then in the resulting table, enter different “Maximum Subjects” for each variant. On the simulations tab FACTS will then create a copy of all the scenarios to run with each variant.