The Summary Results Columns
Highlights
These are the columns displayed on the simulations tab after simulations are completed, they can also be displayed in the separate “Highlights” results window.
| Column Title | Number of columns | Description |
|---|---|---|
| Select | 1 | Not an output column, this column contains check box to allow the user to select which scenario to simulate. The ‘Select All’ button causes them all to be checked. |
| Status | 1 | This column reports on the current status of simulations: Completed, Running, No Results, Out of date, Error. It is updated automatically. |
| Scenario | 1 | This column gives the name of the scenario. It is constructed from the names of all the profiles that have been combined to create the scenario. |
| Num Sims | 1 | The number of simulations that were run to produce the displayed results. |
| Random Number Seed | 1 | Base random number seed used to perform the simulations. |
| Mean Subj. | 1 | This is the mean (over the simulations) of the number of subjects recruited in this scenario. |
| Ppn Success Criteria Met (Final): <group> | One per Group | The proportion of simulations where each groups success criteria have been after final evaluation – for early stopping this means that the Phase 3 and/or CSD criteria, the across groups criteria and the minimum number of subjects to stop were all satisfied. |
| Ppn Study Success (Final) | 1 | The proportion of simulations where the study was a success after final evaluation. |
| Ppn Futility Criteria Met (Final): <group> | One per Group | The proportion of simulations where each groups futility criteria have been met after final evaluation – for early stopping this means that the Phase 3 and/or CSD criteria, the across groups criteria and the minimum number of subjects to stop were all satisfied |
| Ppn Study Futile (Final) | 1 | The proportion of simulations where the study was futile after final evaluation. |
| Ppn Overall Success | 1 | This is the proportion of simulations that stopped for success, either early success or late success (as defined below). |
| Ppn Early Success | 1 | This is the proportion of simulations that stopped early for success (and did not regress to futility in the final analysis – though they might have regressed to ‘inconclusive’). |
| Ppn Late Success | 1 | This is the proportion of simulations that did not stop early but were successful in the final analysis. |
| Ppn Overall Futility | 1 | This is the proportion of simulations that stopped for futility, either early futility or late futility (as defined below). |
| Ppn Late Futility | 1 | This is the proportion of simulations that did not stop early but were futile in the final analysis. |
| Ppn Early Futility | 1 | This is the proportion of simulations that stopped early for futility (and did not regress to success in the final analysis – though they might have regressed to ‘inconclusive’). |
| Ppn Succ to Futility Flipflop | 1 | This is the proportion of simulations that stopped early for success but regressed to futility in the final analysis. |
| Ppn Futility to Succ Flipflop | 1 | This is the proportion of simulations that stopped early for futility but regressed to success in the final analysis. |
| Ppn Inconclusive | 1 | This is the proportion of simulations that did not stop early and were neither successful nor futile in the final analysis. |
| Mean Accrual Duration | 1 | This is the mean (over the simulations) of the duration of the accrual period, from the start of the trial to last patient first visit |
| Ppn Correct Groups | 1 | The proportion of simulations that met the success criteria and selected groups marked as “should succeed” on the Virtual Subject Response > Explicitly Defined > Dose Response tab. For each simulation the fraction of the total number of groups flagged as “Should succeed” is used. |
| Ppn Incorrect Groups | 1 | The proportion of simulations that met the success criteria and selected the arms not marked as “should succeed” on the Virtual Subject Response > Explicitly Defined > Dose Response tab. For each simulation the fraction of the total number of groups not flagged as “Should succeed” is used |
| Version | 1 | This the version number of the FACTS GUI that was used to create the parameters that were passed to the design engine that ran the simulations. |
Allocation
| Column Title | Number of columns | Description |
|---|---|---|
| Scenario | 1 | This column gives the name of the scenario. It is constructed from the names of all the profiles that have been combined to create the scenario. |
| Mean Subj. | 1 | This is the mean (over the simulations) of the number of subjects recruited in this scenario. |
| SD Subj. | 1 | This is the standard deviation across the simulations of the number of subjects recruited. |
| Num Subj 80% | 1 | This is the eightieth percentile across the simulations of the number of subjects recruited into the trial. |
| Mean Alloc.: <Group>, <Arm> | One for each arm in each group | This is the mean (over the simulations) of the number of subjects recruited into each arm in this scenario. |
| Mean Accrual Duration | 1 | This is the mean (over the simulations) of the duration of the accrual period, from the start of the trial to last patient first visit |
Response
| Column Title | Number of columns | Description |
|---|---|---|
| Scenario | 1 | This column gives the name of the scenario. It is constructed from the names of all the profiles that have been combined to create the scenario. |
| Mean Ctrl Response: <Group> | One per group (if control treatment arm included) | These columns are -9999 if there are no control arms included in the design. This is the mean (over the simulations) of the mean estimate of response (change from baseline) for the control arm in each group. |
| Mean Ctrl Response: Across groups | 1 (if control treatment arm included) | This column is -9999 if there are no control arms included in the design. This is the mean (over the simulations) of the average of the response on all the control arms across all the groups. (Note: this is not part of the response model, but estimated separately) |
| Mean Trt Response: <Group> | One per group | This is the mean (over the simulations) of the mean estimate of response (change from baseline) for the study treatment in each group. |
| Mean Trt Response: Across groups | 1 | This is the mean (over the simulations) of the across groups effect size (the estimate across the groups of the difference between response on the study treatment and the historic control rate or the mean response on the control arm). |
| SD Control Resp: <Group> | One per group (if control treatment arm included) | These columns are -9999 if there are no control arms included in the design. This is the standard deviation (over the simulations) of the mean estimate of the response for the control arm in each group. |
| SD Control Resp: Across groups | 1 (if control treatment arm included) | This column is -9999 if there are no control arms included in the design. This is the standard deviation (over the simulations) of the average of the mean estimates of response for all the control arms across the groups. |
| SD Trt Resp: <Group> | One per group | This is the standard deviation (over the simulations) of the mean estimate of the response for the study treatment in each group across the simulations. Note it is not based on the standard deviation estimated in the simulations, it is the SD observed across the simulation results. |
| SD Trt Resp: Across groups | 1 | This is the standard deviation (over the simulations) of the estimate of the across groups effect size. |
| Mean Sigma | 1 | This is the mean (over the simulations) of the mean estimate for ‘sigma’ that is the common standard deviation of the distributions of the final endpoints for each treatment arm. |
| SD Sigma | 1 | This is the standard deviation (across the simulations) of the mean estimates for sigma. |
| Mean Overall Sigma | 1 | This is the mean (over the simulations) of the mean estimate for ‘the across groups sigma’ that is the common standard deviation of the distributions of the final endpoints for the pooled control subjects and pooled study treatment subjects. |
| SD Overall Sigma | 1 | This is the standard deviation (across the simulations) of the mean estimates for the pooled sigma. |
| True Mean Ctrl Resp: <Group> | One per group | This column is -9999 if there are no control arms included in the design. True mean control response for the scenario |
| True SD Ctrl Resp: <Group> | One per group | This column is -9999 if there are no control arms included in the design. This is the true standard deviation of the control response for the scenario. |
| True Mean Trt Resp: <Group> | One per group | True mean treatment response in each group for the scenario. |
| True SD Trt Resp: <Group> | One per group | The standard deviation of the treatment response in each group for the scenario. |
| Mean Mu_Theta | 1 | This is the mean (over the simulations) of the mean estimate of the mean of hierarchical distribution of the mean study treatment difference from control in each group. -9999 if groups are modeled separately. |
| SD Mu_Theta | 1 | This is the standard deviation (over the simulations) of the hierarchical mean of theta. -9999 if groups are modeled separately. |
| Mean Tau_theta | 1 | This is the mean (over the simulations) of the mean estimate of the standard deviation of the hierarchical distribution of the mean study treatment difference from control in each group. -9999 if groups are modeled separately. |
| SD Tau_theta | 1 | This is the standard deviation (across the simulations) of the mean estimate of the standard deviation of the hierarchical distribution of the study treatment difference from control in each group. -9999 if groups are modeled separately. |
| Mean Mu_Gamma | 1 | This is the mean (over the simulations) of the mean estimate of the mean of hierarchical distribution of the mean response on the control arm in each group. -9999 if groups are modeled separately or there is no control. |
| SD Mu_Gamma | 1 | This is the standard deviation (across the simulations) of the mean estimate of the mean of hierarchical distribution of the mean response on the control arm in each group. -9999 if groups are modeled separately or there is no control. |
| Mean Tau_Gamma | 1 | This is the mean (over the simulations) of the mean estimate of the standard deviation of hierarchical distribution of the mean response on the control arm in each group. -9999 if groups are modeled separately or there is no control. |
| SD Tau_Gamma | 1 | This is the standard deviation (across the simulations) of the mean estimate of the standard deviation of hierarchical distribution of the mean response on the control arm in each group. -9999 if groups are modeled separately or there is no control. |
Observed
| Column Title | Number of columns | Description |
|---|---|---|
| Status | 1 | This column reports on the current status of simulations: Completed, Running, No Results, Out of date, Error. It is updated automatically. |
| Scenario | 1 | This column gives the name of the scenario, concatenating together the profile names from the following tabs: ‘Execution > Accrual’, ‘Execution > Dropout Rate’, ‘Virtual Subject Response > Composite’. This is the same name as used for the results directory. |
| Mean Complete <Group> Control | One per group | This is the mean (over the simulations) of the number of subjects recruited per group for the control arm which have had their endpoint observed in this scenario. |
| Mean Complete <Group> Treatment | One per group | This is the mean (over the simulations) of the number of subjects recruited per group for the treatment arm which have had their endpoint observed in this scenario. |
| Mean Complete Info <Group> Control | One per group | This is the mean (over the simulations) of information observed per group for the control arm as defined on the Interims tab (Subjects enrolled, Complete Data at Specified Visit, Opportunity to Complete at Specified Visit) in this scenario. |
| Mean Complete Info <Group> Treatment | One per group | This is the mean (over the simulations) of information observed per group for the treatment arm as defined on the Interims tab (Subjects enrolled, Complete Data at Specified Visit, Opportunity to Complete at Specified Visit) in this scenario. |
Probabilities
| Column Title | Number of columns | Description |
|---|---|---|
| Scenario | 1 | This column gives the name of the scenario. It is constructed from the names of all the profiles that have been combined to create the scenario. |
| Prob. Phase III Success: <Group> | One per group | This is the average (over the simulations) of the probability of success in phase 3 for the study treatment in each group. |
| Prob. Phase III Success: Across groups | 1 | This is the average (over the simulations) of the probability of success in phase 3 for the across groups effect. |
| Prob. CSD (Success): <Group> | One per group | This is the average (over the simulations) of the probability of the response being better than the response on control by the CSD for that group for success. |
| Prob. CSD (Success): Across groups | 1 | This is the average (over the simulations) of the probability of the across group effect being better than the across group CSD for success. |
| Prob. CSD (Futility): <Group> | One per group | This is the average (over the simulations) of the probability of the response being better than the response on control by the CSD for that group for futility. |
| Prob. CSD (Futility): Across groups | 1 | This is the average (over the simulations) of the probability of the across group effect being better than the across group CSD for futility. |
Stopping Rules
| Column Title | Number of columns | Description |
|---|---|---|
| Scenario | 1 | This column gives the name of the scenario. It is constructed from the names of all the profiles that have been combined to create the scenario. |
| Ppn CSD Success Met: <Group> | One per group | This is the proportion of simulations where each group met the CSD success criteria before the final analysis. |
| Ppn CSD Success Met: Across Groups | 1 | This is the proportion of simulations where the across groups analysis met the CSD success criteria before the final analysis. |
| Ppn CSD Futility Met: <Group> | One per group | This is the proportion of simulations where each group met the CSD futility criteria before the final analysis. |
| Ppn CSD Futility Met: Across Groups | 1 | This is the proportion of simulations where the across groups analysis met the CSD futility criteria before the final analysis. |
| Ppn P3 Success Met: <Group> | One per group | This is the proportion of simulations where each group met the probability of success in Phase 3 success criteria before the final analysis. |
| Ppn P3 Success Met: Across Groups | 1 | This is the proportion of simulations where the across group analysis met the probability of success in Phase 3 success criteria before the final analysis. |
| Ppn P3 Futility Met: <Group> | One per group | This is the proportion of simulations where each group met the probability of success in Phase 3 futility criteria before the final analysis. |
| Ppn P3 Futility Met: Across Groups | 1 | This is the proportion of simulations where the across group analysis met the probability of success in Phase 3 futility criteria before the final analysis. |
| Ppn Combined Success: <Group> | One per group | This is the proportion of simulations where each group met both the CSD success criteria and the probability of success in Phase 3 success criteria before the final analysis. |
| Ppn Combined Success: Across groups | 1 | This is the proportion of simulations where the across groups analysis met both the CSD success criteria and the probability of success in Phase 3 success criteria before the final analysis. |
| Ppn Combined Futility: <Group> | One per group | This is the proportion of simulations where each group met both the CSD futility criteria and the probability of success in Phase 3 futility criteria before the final analysis. |
| Ppn Combined Futility: Across groups | 1 | This is the proportion of simulations where the across group analysis met both the CSD futility criteria and the probability of success in Phase 3 futility criteria before the final analysis. |
| Ppn Success Criteria: <Group> | One per group | This is the proportion of simulations where each groups success criteria have been met before the final evaluation, this means that the Phase 3 and/or CSD criteria, the across groups criteria and the minimum number of subjects to stop were all satisfied. |
| Ppn Study Success | 1 | This is the proportion of simulations where the across groups success criteria has been met before the final evaluation, this means that the Phase 3 and/or CSD criteria, and the minimum number of subjects to stop were all satisfied. |
| Ppn Study Success | 1 | This is the proportion of simulations in which the study success criteria were met |
| Ppn Futility Criteria: <Group> | One per group | This is the proportion of simulations where the each groups futility criteria have been met before the final evaluation, this means that the Phase 3 and/or CSD criteria, the across groups criteria and the minimum number of subjects to stop were all satisfied. |
| Ppn Study Futile | 1 | This is the proportion of simulations in which the study futility criteria were met |
Evaluation Rules
| Column Title | Number of columns | Description |
|---|---|---|
| Scenario | 1 | This column gives the name of the scenario. It is constructed from the names of all the profiles that have been combined to create the scenario. |
| Ppn CSD Futility (Final) Met: <Group> | One per group | This is the proportion of simulations where each group met the CSD futility criteria at the final analysis. |
| Ppn CSD Futility (Final) Met: Across Groups | 1 | This is the proportion of simulations where the across groups analysis met the CSD futility criteria at the final analysis. |
| Ppn P3 Futility (Final) Met: <Group> | One per group | This is the proportion of simulations where each group met the probability of success in Phase 3 futility criteria at the final analysis. |
| Ppn P3 Futility (Final) Met: Across Groups | 1 | This is the proportion of simulations where the across group analysis met the probability of success in Phase 3 futility criteria at the final analysis. |
| Ppn CSD Success (Final) Met: <Group> | One per group | This is the proportion of simulations where each group met the CSD success criteria at the final analysis. |
| Ppn CSD Success (Final) Met: Across Groups | 1 | This is the proportion of simulations where the across groups analysis met the CSD success criteria at the final analysis. |
| Ppn P3 Success (Final) Met: <Group> | One per group | This is the proportion of simulations where each group met the probability of success in Phase 3 success criteria at the final analysis. |
| Ppn P3 Success (Final) Met: Across Groups | 1 | This is the proportion of simulations where the across group analysis met the probability of success in Phase 3 success criteria at the final analysis. |
| Ppn Combined Futility (Final): <Group> | One per group | This is the proportion of simulations where each group met both the CSD futility criteria and the probability of success in Phase 3 futility criteria at the final analysis. |
| Ppn Combined Futility (Final): Across groups | 1 | This is the proportion of simulations where the across group analysis met both the CSD futility criteria and the probability of success in Phase 3 futility criteria at the final analysis. |
| Ppn Combined Success (Final): <Group> | One per group | This is the proportion of simulations where each group met both the CSD success criteria and the probability of success in Phase 3 success criteria at the final analysis. |
| Ppn Combined Success (Final): Across groups | 1 | This is the proportion of simulations where the across groups analysis met both the CSD success criteria and the probability of success in Phase 3 success criteria at the final analysis. |
| Ppn Success Criteria Met (Final): <Group> | One per group | This is the proportion of simulations where each groups success criteria have been met at the final evaluation, this means that the Phase 3 and/or CSD criteria and any across groups criteria were all satisfied. |
| Ppn Study Success (Final) | 1 | This is the proportion of simulations where the across groups success criteria has been met at the final evaluation, this means that the Phase 3 and/or CSD criteria were all satisfied. |
| Ppn Study Success | 1 | This is the proportion of simulations in which the study success criteria were met at the final evaluation |
| Ppn Futility Criteria Met (Final): <Group> | One per group | This is the proportion of simulations where the each groups futility criteria have been met at the final evaluation, this means that the Phase 3 and/or CSD criteria, and any across groups criteria were all satisfied. |
| Ppn Study Futile (Final) | 1 | This is the proportion of simulations in which the study futility criteria were met at the final evaluation |
Hierarchical Prior Parameters
| Column Title | Number of columns | Description |
|---|---|---|
| Scenario | 1 | This column gives the name of the scenario. It is constructed from the names of all the profiles that have been combined to create the scenario. |
| Mean BAC Mu: <Group> | One per group | This is the mean (over the simulations) of the mean estimate of the mean of the hierarchical distribution of the response on the control arm in the group and the response on the control arms in the specified historical studies. -9999 if BAC not used. |
| SD BAC Mu: <Group> | One per group | This is the standard deviation (over the simulations) of the mean estimate of the mean of the hierarchical distribution of the response on the control arm in the group and the response on the control arms in the specified historical studies. -9999 if BAC not used. |
| Mean Bac Tau: <Group> | One per group | This is the mean (over the simulations) of the mean estimate of the standard deviation of the hierarchical distribution of the response on the control arm in the group and the response on the control arms in the specified historical studies. -9999 if BAC not used. |
| SD Bac Tau: <Group> | One per group | This is the standard deviation (over the simulations) of the mean estimate of the standard deviation of the hierarchical distribution of the response on the control arm in the group and the response on the control arms in the specified historical studies. -9999 if BAC not used. |
Baseline
| Column Title | Number of columns | Description |
|---|---|---|
| Scenario | 1 | This column gives the name of the scenario. It is constructed from the names of all the profiles that have been combined to create the scenario. |
| Mean Baseline Beta | 1 | This is the average (over the simulations) of the β that relates baseline to subject response. Values are -9999 if baseline adjustment not used. |
| SD Baseline Beta | 1 | This is the standard deviation (over the simulations) of the β that relates baseline to subject response. Values are -9999 if baseline adjustment not used. |
| Mean Baseline: <Group> | One per group | This is the average (over the simulations) of the baseline. Values are -9999 if baseline is not used |
| SD Baseline <Group> | One per group | This is the standard deviation (over the simulations) of the baseline. Values are -9999 if baseline is not used |
| True Mean Baseline <Group> | One per group | True mean baseline for the scenario |
| True SD Baseline <Group> | One per group | True standard deviation of baseline for the scenario |
Model Parameters
These parameters are unused in ED unless the Time Course Hierarchical or ITP longitudinal analysis models are used.
| Column Title | Number of columns | Description |
|---|---|---|
| Scenario | 1 | This column gives the name of the scenario. It is constructed from the names of all the profiles that have been combined to create the scenario. |
| Mean Longmod Resp: <Group> <Arm> <Visit> | Group * Arms * Visits | This is the mean (over the simulations) of the estimate of the mean response for that particular arm, in that group at that visit. |
| SE Mean Longmod Resp: <Group> <Arm> < Visit> | Group * Arms * Visits | This is the Standard Error (over the simulations) of the estimate of the mean response for that particular arm, in that group at that visit. |
Simulation Results
This is a display of the contents of the simulations.csv files, with windows available via the right click menu that show various relevant column groupings similar to the summary results.
Frequentist Results
| Column Title | Number of columns | Description |
|---|---|---|
| Scenario | 1 | This column gives the name of the scenario. It is constructed from the names of all the profiles that have been combined to create the scenario. |
| Ppn Signif (Grp) | 1 | This is the proportion of simulations where the difference in response between groups was significant |
| Mean Overall Trt Effect | 1 | This is the mean (over the simulations) of the frequentist estimate of the overall treatment effect |
| SE Mean Overall Trt Effect | 1 | This is the Standard Error (over the simulations) of the frequentist estimate of the overall treatment effect |
| Ppn Signif (Trt) | 1 | This is the proportion of the simulations where the overall difference between treatment and control was significant |
| Ppn Signif (Grp x Trt) | 1 | This is the proportion of the simulations where the treatment x group interaction term was significant. |
| Mean Trt Effect: <Group> | One per group | This is the mean (over the simulations) of the frequentist estimate of the treatment effect within each group. |
| SD Trt Effect: <Group> | One per group | This is the SD (over the simulations) of the frequentist estimate of the treatment effect within each group. |
| Mean Sigma | 1 | This is the mean (over the simulations) of the frequentist estimate of the SD of the response across all subjects. |
| SD Sigma | 1 | This is the SD (over the simulations) of the frequentist estimate of the SD of the response across all subjects. |
| Mean Beta | 1 | This is the mean (over the simulations) of the estimate of the regression coefficient for the baseline. |
| SD Beta | 1 | This is the SD (over the simulations) of the estimate of the regression coefficient for the baseline. |
| PPn Signif: <Group> | One per group | This is the proportion of the simulations where the within group difference between treatment and control was significant. |
Frequentist Ancova Results
The columns in this table are the same as for the “Frequentist” results, but are the results of an ANCOVA test.
Output Files
FACTS stores the results of simulations as ‘.csv’ files under a Results folder. For each row in the simulations table, there is a folder named by the profiles that make up the scenario, which contains the corresponding ‘.csv’ files. Right-clicking on the row displays a context menu which includes the option to open explore in the corresponding folder.
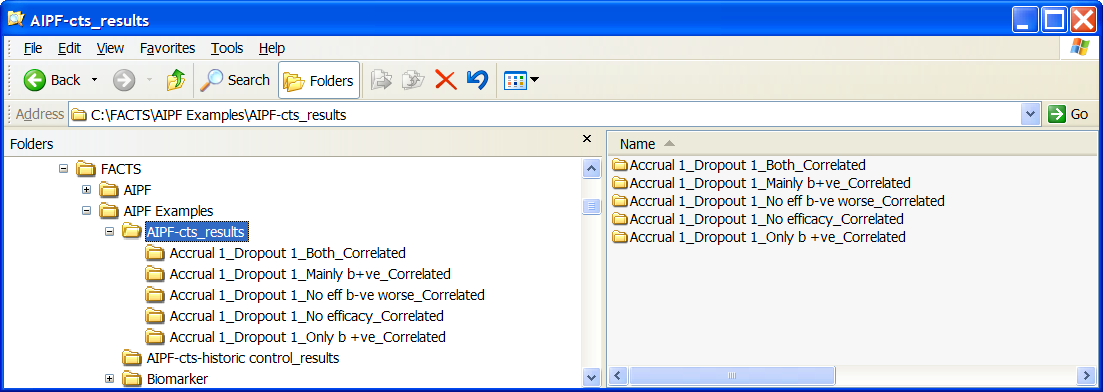
These files can be opened using Microsoft Excel, but versions of Excel before 2007 are restricted to 256 columns, which is too few to view some files in their entirety. The ‘Calc’ application in ‘OpenOffice’ will show all the columns (and will open two files that have the same name at the same time!). Because Excel takes out a file lock on any file it has open, while a file is open in Excel it cannot be deleted or modified by another application. The most common cause for an error to be reported when simulating trials in FACTS is because the user has one of the previous results files is still open in Excel.
In the scenario directory there are the following types of results file:
Summary.csv Contains a single row of data that summarizes the simulation results. This is the source of the shown on the simulations tab.
Summary_freq_bocf.csv
Summary_freq_fail.csv
Summary_freq_ignore.csv
Summary_freq_locf.csv Contains a single row of data that that summarizes the frequentist analysis of the simulations. A file is output for each of the different methods for dealing with missing data that are applicable: BOCF if the baseline score is being simulated (Continuous Only), LOCF and “ignore” (referred to as PP (Per-Protocol) in the GUI) and Fail (Dichotomous only).
Simulations.csv Contains one row per simulation describing the final state of each simulation for every trial simulated.
Simulations_freq_bocf.csv
Simulations_freq_fail.csv
Simulations_freq_ignore.csv
Simulations_freq_locf.csv Contains one row per simulation describing the frequentist analysis of the final state of each simulation for every trial simulated. A file is output for each of the different methods for dealing with missing data that are applicable: BOCF if the baseline score is being simulated (Continuous only), LOCF and “ignore” (referred to as PP (Per-Protocol) in the GUI) and Fail (Dichotomous only).
PatientsNNNNN.csv Contains one row per patient in simulation, where NNNNN is the number of the simulation. By default this file is written out only for the first simulation, but this can be changed via the ‘Advanced’ button on the simulations tab.
WeeksNNNNN.csv Contains one row for each interim during a simulation where NNN is the number of the simulation. By default this file is written only for the first 100 simulations, but this can be changed via the simulation tab. The values in the last row of the “weeks” file will be the same as the final values for that simulation in the simulations file.
weeks_freq_bocf_NNNNN.csv,
weeks_freq_fail_NNNN.csv,
weeks_freq_ignore_NNNN.csv,
weeks_freq_locf_NNNNN.csv,
Contains one row for each interim during a simulation where NNNNN is the number of the simulation. A file is output for each simulation for which a frequentist “weeks file” is to be output, and for each of the different methods for dealing with missing data that are applicable: BOCF if the baseline score is being simulated (Continuous Only), LOCF and “ignore” (referred to as PP (Per-Protocol) in the GUI) and Fail (Dichotomous only)
Contents of summary.csv
The columns in summary.csv are common across all the FACST Dose Finding design engines, hence there are columns in the file when using the N-CRM design engine with no contents as they are for results no applicable to this engine.
| Column Title | Number of columns | Description |
|---|---|---|
| # Project | 1 | The name of the “.facts” file in the FACTS GUI that was used to generate the simulations. |
| Scenario | 1 | The name of the scenario – This is the various profile names that make up the scenario, concatenated together. |
| Timestamp | 1 | The date and time when the simulations started. |
| Version | 1 | The version number of the FACTS GUI that ran the simulations. |
| Nsim | 1 | The number of simulation runs. |
| No. Subj | 1 | The mean, over the simulations, of the total number of subjects recruited in the trial. |
| SE Subj. | 1 | The standard error of the total number of subjects recruited into the trials. |
| 80-ile | 1 | The 80th percentile, over the simulations, of the total number of subjects recruited in the trial. |
| Mean Alloc <Group> |
2*G | The mean number of subjects recruited into each arm in each group. If a control arm is not included the column is still present, with value ‘0’. |
| Mean Trt Resp <Group> | G | The mean of the mean estimates of response on the study treatment arm in each group. |
| Mean Trt Effect (Overall) | 1 | The mean of the mean estimate of the across groups treatment difference. |
| SE Trt Resp <Group> | G | The standard error, over the simulations, of the mean estimate of response on the study treatment arm in each group. |
| SE Trt Effect (Overall) | 1 | The standard error, over the simulations, of the mean estimate of the across groups treatment difference. |
| Mean Control Resp <Group> | G | The mean of the mean estimates of response on the control arm in each group. |
| Avg. Control Resp (Overall) | 1 | The mean of the average of the control response across the groups |
| SE Control Resp <Group> | G | The standard error, over the simulations, of the mean estimate of response rate on the control arm in each group. |
| SE Avg. Control Effect (Overall) | 1 | The standard error, over the simulations, of the mean estimate of the across groups control response rate. |
| Mean Sigma | 1 | The mean of the estimate of the standard deviation in the endpoint from the estimates of the response of the individual arms in each group. |
| SE Sigma | 1 | The standard error of the estimate of the standard deviation in the endpoint from the estimates of the response of the individual arms in each group. |
| Mean Overall Sigma | 1 | The mean of the estimate of the standard deviation in the endpoint from the estimates of the treatment difference across the groups. |
| SE Overall Sigma | 1 | The standard error of the estimate of the standard deviation in the endpoint from the estimates of the treatment difference across the groups. |
| True Mean Trt Resp <Group> | G | True mean treatment response for the scenario |
| True Mean Control Resp <Group> | G | True mean control response for the scenario |
| Mean Baseline Beta | 1 | The mean of the mean estimates of the baseline adjustment |
| SE Baseline Beta | 1 | The standard error of the mean estimates of the baseline adjustment |
| Mean Baseline <Group> | G | The mean of the mean estimate of the baseline |
| SE Baseline <Group> | G | The standard error of the mean estimates of the baseline |
| SD Baseline <Group> | G | The mean of the SDs of the estimates of the baseline |
| SE SD Baseline <Group> | G | The standard error of the estimates of the SD of the baseline |
| True Mean Baseline <Group> | G | The true mean baseline from the scenario |
| True SD Baseline <Group> | G | The true standard deviation of the baseline for the scenario |
| Mean Mu Theta | 1 | The mean, over the simulations, of the mean estimate of the mean of hierarchical distribution of the mean study treatment difference from control in each group. |
| SE Mu Theta | 1 | The standard error, over the simulations, of the mean estimate of the mean of the hierarchical distribution of the mean study treatment difference from control in each group. |
| Mean Tau Theta | 1 | The mean, over the simulations, of the mean estimate of the standard deviation of the hierarchical distribution of the mean study treatment difference from control in each group. |
| SE Tau Theta | 1 | The standard error, across the simulations, of the mean estimate of the standard deviation of the hierarchical distribution of the study treatment difference from control in each group. |
| Mean Mu Gamma | 1 | The mean, over the simulations, of the mean estimate of the mean of hierarchical distribution of the mean response on the control arm in each group. |
| SE Mu Gamma | 1 | The standard error, over the simulations, of the mean estimate of the mean of the hierarchical distribution of the mean response on the control arm in each group. |
| Mean Tau Gamma | 1 | The mean, over the simulations, of the mean estimate of the standard deviation of hierarchical distribution of the mean response on the control arm in each group. |
| SE Tau Gamma | 1 | The standard error, across the simulations, of the mean estimate of the standard deviation of hierarchical distribution of the mean response on the control arm in each group. |
| Mean Longmod Resp <Group> |
G * A * V | This is the mean, over the simulations, of the response in a particular group, on a particular arm at a particular visit. These columns are only present if a TCH or ITP longitudinal model is being fitted (other models do not produce an estimate of the mean response at a visit). |
| SE Mean Longmod Resp <Group> |
G * A *V | This is the Standard Error, over the simulations, of the mean estimate of response at a visit. These columns are only present if a TCH or ITP longitudinal model is being fitted (other models do not produce an estimate of the mean response at a visit). |
| Mean Pr Ph3 Success <Group> | G | The mean, over the simulations, of the mean probability of success in phase 3 of the study treatment in each group. |
| Mean Pr Ph3 Success 99 | 1 | The mean, across the simulations, of the mean probability of success in phase 3 of the across groups treatment difference. |
| Mean Pr CSD (Success) <Group> | G | The mean, over the simulations, of the mean probability of the study treatment arm being better than the control by the CSD for success in each group. |
| Mean Pr CSD (Success) 99 | 1 | The mean, over the simulations, of the mean probability of the across groups treatment difference being better than the CSD for success. |
| Mean Pr CSD (Futility) <Group> | G | The mean, over the simulations, of the mean probability of the study treatment arm being better than the control by the CSD for futility in each group. |
| Mean Pr CSD (Futility) 99 | 1 | The mean, over the simulations, of the mean probability of the across groups treatment difference being better than the CSD for futility. |
| Ppn CSD Futility <Group> | G | The proportion of simulations in which the estimate of the response on the study treatment arm met its CSD futility criterion in each group. |
| Ppn CSD Futility 99 | 1 | The proportion of simulations in which the across group treatment difference met its CSD futility criterion. |
| Ppn Ph3 Futility <Group> | G | The proportion of simulations in which the predicted probability of success in phase 3 of the response on the study treatment arm met its futility criterion in each group. |
| Ppn Ph3 Futility 99 | 1 | The proportion of simulations in which the across group treatment difference met its probability of success in phase 3 futility criterion. |
| Ppn CSD Success <Group> | G | The proportion of simulations in which the estimate of the response on the study treatment arm met its CSD success criterion in each group. |
| Ppn CSD Success 99 | 1 | The proportion of simulations in which the across group treatment difference met its CSD success criterion. |
| Ppn Ph3 Success <Group> | G | The proportion of simulations in which the predicted probability of success in phase 3 of the response on the study treatment arm met its success criterion in each group. |
| Ppn Ph3 Success 99 | 1 | The proportion of simulations in which the across group treatment difference met its probability of success in phase 3 success criterion. |
| Ppn Combined Futility <Group> | G | The proportion of simulations where each group met both the CSD futility criteria and the probability of success in Phase 3 futility criteria. |
| Ppn Combined Futility 99 | 1 | The proportion of simulations where the across groups treatment effect met both the CSD futility criteria and the probability of success in Phase 3 futility criteria. |
| Ppn Combined Success <Group> | G | The proportion of simulations where each group met both the CSD success criteria and the probability of success in Phase 3 success criteria. |
| Ppn Combined Success 99 | 1 | The proportion of simulations where the across groups treatment effect met both the CSD success criteria and the probability of success in Phase 3 success criteria. |
| Ppn Success Criteria Met <Group> | G | The proportion of simulations where each groups success criteria have been met either for early stopping or final evaluation – for early stopping this means that the Phase 3 and/or CSD criteria, the across groups criteria and the minimum number of subjects to stop were all satisfied. |
| Ppn Study Success | 1 | The proportion of simulations where the study was a success (whether stopping early or reaching full accrual). |
| Ppn Futility Criteria Met <Group> | G | The proportion of simulations where each groups futility criteria have been met either for early stopping or final evaluation – for early stopping this means that the Phase 3 and/or CSD criteria, the across groups criteria and the minimum number of subjects to stop were all satisfied |
| Ppn Study Futile | 1 | The proportion of simulations where the study was futile (whether stopping early or reaching full accrual). |
| Ppn CSD Futility (Final) <Group> | G | The proportion of simulations in which the estimate after all data has been collected of the response on the study treatment arm met its CSD futility criterion in each group. |
| Ppn CSD Futility (Final) 99 | 1 | The proportion of simulations in which after all data has been collected the across group treatment difference met its CSD futility criterion. |
| Ppn Ph3 Futility (Final) <Group> | G | The proportion of simulations in which the predicted probability after all data has been collected of success in phase 3 of the response on the study treatment arm, met its futility criterion in each group. |
| Ppn Ph3 Futility (Final) 99 | 1 | The proportion of simulations in which the across group treatment difference after all data has been collected met its probability of success in phase 3 futility criterion. |
| Ppn CSD Success (Final) <Group> | G | The proportion of simulations in which the estimate after all data has been collected of the response on the study treatment arm met its CSD success criterion in each group. |
| Ppn CSD Success (Final) 99 | 1 | The proportion of simulations in which the across group treatment difference after all data has been collected met its CSD success criterion. |
| Ppn Ph3 Success (Final) <Group> | G | The proportion of simulations in which the predicted probability after all data has been collected of success in phase 3 of the response on the study treatment arm, met its success criterion in each group. |
| Ppn Ph3 Success (Final) 99 | 1 | The proportion of simulations in which the across group treatment difference after all data has been collected met its probability of success in phase 3 success criterion. |
| Ppn Combined Futility (Final) <Group> | G | The proportion of simulations where after all data has been collected each group met both the CSD futility criteria and the probability of success in Phase 3 futility criteria. |
| Ppn Combined Futility (Final) 99 | 1 | The proportion of simulations where the across groups treatment effect after all data has been collected met both the CSD futility criteria and the probability of success in Phase 3 futility criteria. |
| Ppn Combined Success (Final) <Group> | G | The proportion of simulations where after all data has been collected each group met both the CSD success criteria and the probability of success in Phase 3 success criteria. |
| Ppn Combined Success (Final) 99 | 1 | The proportion of simulations where after all data has been collected the across groups treatment effect met both the CSD success criteria and the probability of success in Phase 3 success criteria. |
| Ppn Success Criteria Met (Final) <Group> | G | The proportion of simulations where after all data has been collected each groups success criteria have been met either for early stopping or final evaluation – for early stopping this means that the Phase 3 and/or CSD criteria, the across groups criteria and the minimum number of subjects to stop were all satisfied. |
| Ppn Study Success (Final) | 1 | The proportion of simulations where after all data has been collected the study was a success (whether stopping early or reaching full accrual). |
| Ppn Futility Criteria Met (Final) <Group> | G | The proportion of simulations where after all data has been collected each groups futility criteria have been met either for early stopping or final evaluation – for early stopping this means that the Phase 3 and/or CSD criteria, the across groups criteria and the minimum number of subjects to stop were all satisfied |
| Ppn Study Futile (Final) | 1 | The proportion of simulations after all data has been collected where the study was futile (whether stopping early or reaching full accrual). |
| Ppn Outcome 1 | 1 | The proportion of simulations that stopped early for success. |
| Ppn Outcome 2 | 1 | The proportion of simulations that reached full accrual and declared success on final evaluation. |
| Ppn Outcome 3 | 1 | The proportion of simulations that reached full accrual and declared futility on final evaluation. |
| Ppn Outcome 4 | 1 | The proportion of simulations that stopped early for futility |
| Ppn Outcome 5 | 1 | The proportion of simulations that stopped early for success but were deemed futile on final evaluation. |
| Ppn Outcome 6 | 1 | The proportion of simulations that stopped early for futility but were deemed successful on final evaluation. |
| Ppn Outcome 7 | 1 | The proportion of simulations that reached full accrual and were inconclusive. |
| Mean Study Accrual Stop Week | 1 | The mean study duration of accrual – from start of accrual to last patient first visit. |
| Mean BAC Mu <Group> | G | The mean, over the simulations, of the posterior mean of the Bayesian Augmented Control hierarchical distribution for each group. |
| SE BAC Mu <Group> | G | The standard error, over the simulations, of the posterior mean of the Bayesian Augmented Control hierarchical distribution for each group. |
| Mean BAC Tau <Group> | G | The mean, over the simulations, of the posterior standard deviation of the Bayesian Augmented Control hierarchical distribution for each group. |
| SE BAC Tau <Group> | G | The standard error, over the simulations, of the posterior standard deviation of the Bayesian Augmented Control hierarchical distribution for each group. |
Contents of Summary_freq.csv
| Column Title | Number of columns | Description |
|---|---|---|
| Ppn Significant (Grp) | 1 | The proportion of simulations with a significant group effect. |
| Mean Overall Trt Effect | 1 | This is the mean (over the simulations) of the frequentist estimate of the overall treatment effect |
| SE Mean Overall Trt Effect | 1 | This is the Standard Error (over the simulations) of the frequentist estimate of the overall treatment effect |
| Ppn Significant (Trt) | 1 | The proportion of simulations with an overall significant treatment effect. |
| significant (GrpxTrt) | 1 | The proportion of simulations where the group interaction term was significant. |
| Mean Trt Effect <group> | G | The mean, over the simulations, of the estimate of the mean treatment effect in each group. |
| SE Trt Effect <group> | G | The standard error, over the simulations, of the estimate of the mean treatment effect in each group |
| Mean Sigma | 1 | The mean, over the simulations, of the estimate of the standard deviation in the final response. |
| SE Sigma | 1 | The standard error, over the simulations, of the estimate of the standard deviation in the final response. |
| Mean Beta | 1 | This is the mean (over the simulations) of the estimate of the regression coefficient for the baseline. |
| SE Beta | 1 | This is the SD (over the simulations) of the estimate of the regression coefficient for the baseline. |
| Ppn Significant <group> | G | The proportion of simulations in the which the treatment effect was significant in each group. |
Contents of simulations.csv and weeksNNNNN.csv
Most of the columns are common to the two file types, but the weeks file does not contain columns for the ‘final’ values of the evaluation criteria.
| Column Title | Number of columns | In simulations file | In weeks file | Description |
|---|---|---|---|---|
| #Sims | 1 | ✔ | Simulation number | |
| Weeks (Duration) | 1 | ✔ | The week of final analysis – the total duration of the simulation. | |
| #Week | 1 | ✔ | Week | |
| No. Subj | ✔ | ✔ | The number of subjects recruited in the simulation. | |
| Alloc <group> <arm> | 2 * G | ✔ | ✔ | The number of subjects allocated to each arm in each group. |
| Mean Trt Resp <group> | G | ✔ | ✔ | The estimated mean response of the study treatment in the group. |
| Trt Effect (Overall) | 1 | ✔ | ✔ | The estimated mean treatment difference across the groups. |
| SD Mean Trt Resp <group> | G | ✔ | ✔ | The standard deviation of the estimate of the mean response of the study treatment in the group. |
| SD Trt Effect (Overall) | 1 | ✔ | ✔ | The standard deviation of the estimate of the mean treatment difference across the groups. |
| Mu Theta | 1 | ✔ | ✔ | The mean of the hierarchical distribution of the treatment differences over all the groups. |
| Tau Theta | 1 | ✔ | ✔ | The standard deviation of the hierarchical distribution of the treatment differences over all the groups. |
| Mean Control Resp <group> | G | ✔ | ✔ | The estimated mean response of the control arm in each group. |
| Avg. Control Resp (Overall) | 1 | ✔ | ✔ | The average overall mean response of the control arm over all the groups. (Note: this is not part of the response model, but estimated separately). |
| SD Mean Control Resp <group> | G | ✔ | ✔ | The standard deviation of the estimate of the mean response of the control arm in each group. |
| SD Avg. Control Resp (Overall) | 1 | ✔ | ✔ | The standard deviation of the estimate of the average response over all the control arms. |
| Mu Gamma | 1 | ✔ | ✔ | The mean of the hierarchical distribution of the responses on the control arms across all the groups. |
| Tau Gamma | 1 | ✔ | ✔ | The standard deviation of the hierarchical distribution of the responses on the control arms across all the groups. |
| Sigma | 1 | ✔ | ✔ | The estimate of the standard deviation of the final responses given the per-group response model. |
| SD Sigma | 1 | ✔ | ✔ | The standard deviation of the estimate of the standard deviation of the final responses given the per-group response model. |
| Sigma Overall | 1 | ✔ | ✔ | The estimate of the standard deviation of the final response given the across-groups response model. |
| SD Sigma Overall | 1 | ✔ | ✔ | The standard deviation of the estimate of the standard deviation of the final response given the across group response model. |
| True Mean Trt Resp <group> | G | ✔ | ✔ | True mean treatment response for the scenario |
| True SD Trt Resp <group> | G | ✔ | ✔ | True standard deviation of treatment response for the scenario |
| True Mean Control Resp <group> | G | ✔ | ✔ | True mean control response for the scenario |
| True SD Control Resp <group> | G | ✔ | ✔ | True standard deviation of control response for the scenario |
| Baseline Beta | 1 | ✔ | ✔ | The estimated mean of the baseline adjustment |
| SD Baseline Beta | 1 | ✔ | ✔ | The estimated standard deviation of the baseline adjustment |
| Mean Baseline <group> | G | ✔ | ✔ | The estimated mean of the baseline |
| SE Baseline <group> | G | ✔ | ✔ | The estimated standard error of the baseline |
| SD Baseline <group> | G | ✔ | ✔ | The estimated mean of the standard deviation of baseline |
| True Mean Baseline <group> | G | ✔ | ✔ | True mean baseline from the scenario |
| True SD Baseline <group> | G | ✔ | ✔ | True standard deviation of baseline from the scenario |
| Num Completed <group> <arm> | 2 * G | ✔ | ✔ | The number of subjects completed (final endpoint available) in each arm in each group. |
| Complete Info <group> <arm> | 2 * G | ✔ | ✔ | The amount of complete information in each arm in each group – however that has been defined in the definition of interim timing – subjects enrolled, subjects complete at a certain visit or subjects who had the opportunity to complete at a certain visit. |
| Mean Raw Response <group> <arm> | 2 * G | ✔ | ✔ | The mean raw response |
| SD Mean Raw Response <group> <arm> | 2 * G | ✔ | ✔ | The standard deviation of the raw response |
| Num Dropouts <group> <arm> | 2 * G | ✔ | ✔ | Number of dropouts seen |
| Pr Ph3 Success <group> | G | ✔ | ✔ | The probability of success in phase 3 of the study treatment for each group. |
| Pr Ph3 Success 99 | 1 | ✔ | ✔ | The probability of success in phase 3 of the across groups treatment difference. |
| Pr CSD (Success) <group> | G | ✔ | ✔ | The probability of being better than control by the success CSD (or as good as control by the success NIM) of the study treatment for each group. |
| Pr CSD (Success) 99 | 1 | ✔ | ✔ | The probability of being better than control by the success CSD (or as good as control by the NIM) of the across groups treatment difference. |
| Pr CSD (Futility) <group> | G | ✔ | ✔ | The probability of being better than control by the futility CSD (or as good as control by the futility NIM) of the study treatment for each group. |
| Pr CSD (Futility) 99 | 1 | ✔ | ✔ | The probability of being better than control by the futility CSD (or as good as control by the futility NIM) of the across groups treatment difference. |
| CSD Futility <group> | G | ✔ | ✔ | A flag: 1 = the posterior probability of being better than control by the futility CSD was below the threshold for early stopping for futility for each group, 0 = the posterior probability was above the threshold. |
| CSD Futility 99 | 1 | ✔ | ✔ | A flag: 1 = the posterior probability of being better than control by the futility CSD was below the threshold for early stopping for futility for the across groups analysis, 0 = the posterior probability was above the threshold. |
| Ph3 Futility <group> | G | ✔ | ✔ | A flag: 1 = the posterior probability of successful in a subsequent phase 3 trial was below the threshold for early stopping for futility for each group, 0 = the posterior probability was above the threshold. |
| Ph3 Futility 99 | 1 | ✔ | ✔ | A flag: 1 = the posterior probability of successful in a subsequent phase 3 trial was below the threshold for early stopping for futility in the across groups analysis, 0 = the posterior probability was above the threshold. |
| CSD Success <group> | G | ✔ | ✔ | A flag: 1 = the posterior probability of being better than control by the success CSD was above the threshold for early stopping for success for each group, 0 = the posterior probability was below the threshold. |
| CSD Success 99 | 1 | ✔ | ✔ | A flag: 1 = the posterior probability of being better than control by the success CSD was above the threshold for early stopping for success in the across groups analysis, 0 = the posterior probability was below the threshold. |
| Ph3 Success <group> | G | ✔ | ✔ | A flag: 1 = the posterior probability of successful in a subsequent phase 3 trial was above the threshold for early stopping for success for each group, 0 = the posterior probability was below the threshold. |
| Ph3 Success 99 | 1 | ✔ | ✔ | A flag: 1 = the posterior probability of successful in a subsequent phase 3 trial was above the threshold for early stopping for success in the across groups analysis, 0 = the posterior probability was below the threshold. |
| Combined Futility <group> | G | ✔ | ✔ | A flag: 1 = the required CSD, success in phase 3 and minimum sample size criteria are all met for early stopping for futility for each group, 0 = they are not all met. Whether the across group stopping condition is also met if required is not included in this flag. |
| Combined Futility 99 | 1 | ✔ | ✔ | A flag: 1 = the required CSD, success in phase 3 and minimum sample size criteria are all met for early stopping for futility in the across groups analysis, 0 = they are not all met. |
| Combined Success <group> | G | ✔ | ✔ | A flag: 1 = the required CSD, success in phase 3 and minimum sample size criteria are all met for early stopping for success for each group, 0 = they are not all met. Whether the across group stopping condition is also met if required is not included in this flag. |
| Combined Success 99 | 1 | ✔ | ✔ | A flag: 1 = the required CSD, success in phase 3 and minimum sample size criteria are all met for early stopping for success in the across groups analysis, 0 = they are not all met. |
| Success Criteria Met <group> | G | ✔ | ✔ | A flag: 1 = the required success criteria were met. 0 = Not met. |
| Success Criteria Met 99 | 1 | ✔ | ✔ | A flag: 1 = the required success criteria were met. 0 = Not met. |
| Futility Criteria Met <group> | G | ✔ | ✔ | A flag: 1 = the required futility criteria were met. 0 = Not met. |
| Futility Criteria Met 99 | 1 | ✔ | ✔ | A flag: 1 = the required futility criteria were met. 0 = Not met. |
| CSD Futility (Final) <group> | G | ✔ | A flag: 1 = the posterior probability of being better than control by the futility CSD was below the threshold for the final evaluation for futility for each group, 0 = the posterior probability was above the threshold. | |
| CSD Futility (Final) 99 | 1 | ✔ | A flag: 1 = the posterior probability of being better than control by the futility CSD was below the threshold for the final evaluation for futility in the across groups analysis, 0 = the posterior probability was above the threshold. | |
| Ph3 Futility (Final) <group> | G | ✔ | A flag: 1 = the posterior probability of successful in a subsequent phase 3 trial was below the threshold for the final evaluation for futility for each group, 0 = the posterior probability was above the threshold. | |
| Ph3 Futility (Final) 99 | 1 | ✔ | A flag: 1 = the posterior probability of successful in a subsequent phase 3 trial was below the threshold for the final evaluation for futility in the across groups analysis, 0 = the posterior probability was above the threshold. | |
| CSD Success (Final) <group> | G | ✔ | A flag: 1 = the posterior probability of being better than control by the success CSD was above the threshold for the final evaluation for success for each group, 0 = the posterior probability was below the threshold. | |
| CSD Success (Final) 99 | 1 | ✔ | A flag: 1 = the posterior probability of being better than control by the success CSD was above the threshold for the final evaluation for success in the across groups analysis, 0 = the posterior probability was below the threshold. | |
| Ph3 Success (Final) <group> | G | ✔ | A flag: 1 = the posterior probability of successful in a subsequent phase 3 trial was above the threshold for the final evaluation for success for each group, 0 = the posterior probability was below the threshold. | |
| Ph3 Success (Final) 99 | 1 | ✔ | A flag: 1 = the posterior probability of successful in a subsequent phase 3 trial was above the threshold for the final evaluation for success in the across groups analysis, 0 = the posterior probability was below the threshold. | |
| Combined Futility (Final) <group> | G | ✔ | A flag: 1 = the required CSD and success in phase 3 criteria are met for the final evaluation for futility for each group, 0 = they are not all met. Whether the across group final futility condition is also met if required is not included in this flag. | |
| Combined Futility (Final) 99 | 1 | ✔ | A flag: 1 = the required CSD and success in phase 3 criteria are all met for the final evaluation for futility in the across group analysis, 0 = they are not all met. | |
| Combined Success (Final) <group> | G | ✔ | A flag: 1 = the required CSD and success in phase 3 criteria are all met for the final evaluation for success for each group, 0 = they are not all met. Whether the across group final success condition is also met if required is not included in this flag. | |
| Combined Success (Final) 99 | 1 | ✔ | A flag: 1 = the required CSD and success in phase 3 criteria are all met for the final evaluation for success in the across groups analysis, 0 = they are not all met. | |
| Success Criteria Met (Final) <group> | G | ✔ | A flag: 1 = the group’s success criteria were met, 0 = otherwise. | |
| Success Criteria Met (Final) 99 | 1 | ✔ | A flag: 1 = the group’s success criteria were met, 0 = otherwise. | |
| Futility Criteria Met (Final) <group> | G | ✔ | A flag: 1 = the group’s futility criteria were met, 0 = otherwise. | |
| Futility Criteria Met (Final) 99 | 1 | ✔ | A flag: 1 = the group’s futility criteria were met, 0 = otherwise. | |
| Futile Study | 1 | ✔ | ✔ | A flag: 1 = the study was futile overall, 0 = otherwise. |
| Successful Study | 1 | ✔ | ✔ | A flag: 1 = the study was successful overall, 0 = otherwise. |
| Outcome | 1 | ✔ | ✔ | A flag categorizing final study outcome: = Early success = Late success = Late futility = Early futility = Success to futility flip-flop = Futility to success flip-flop = Inconclusive |
| Group Outcome <group> | G | ✔ | ✔ | A flag categorizing the group outcome: = Early success = Late success = Late futility = Early futility = Success to futility flip-flop = Futility to success flip-flop = Inconclusive |
| Group Stop Type <group> | G | ✔ | ✔ | A flag categorizing the group outcome: = Success = Group Cap = Futility = Other (group stopped because study stopped early) = Study cap |
| Group Stop Week <group> | G | ✔ | ✔ | The week the group stop decision was taken. There may be further follow-up time before the group analysis was completed. |
| Accrual Stop Week | 1 | ✔ | ✔ | The week the study stop decision was taken. There may be further follow-up time before the study analysis was completed. |
| BAC Mu <group> | G | ✔ | ✔ | The mean estimated value of the mean of the Bayesian Augmented Control hierarchical distribution for each group. |
| SD BAC Mu <group> | G | ✔ | ✔ | The standard deviation of the estimate of the mean of the Bayesian Augmented Control hierarchical distribution for each group. |
| BAC Tau <group> | G | ✔ | ✔ | The mean estimated value of the standard deviation of the Bayesian Augmented Control hierarchical distribution for each group. |
| SD BAC Tau <group> | G | ✔ | ✔ | The standard deviation of the estimate of the standard deviation of the Bayesian Augmented Control hierarchical distribution for each group. |
| Linear Regression Alpha <model> <visit> | LM*V | ✔ | ✔ | Only if using the linear regression longitudinal model the mean estimate of the constant offset in the change in response from this visit to the final visit |
| Linear Regression Beta <model> <visit> | LM*V | ✔ | ✔ | Only if using the linear regression longitudinal model the mean estimate of the coefficient of change in response from this visit to the final visit |
| Linear Regression Lambda <model> <visit> | LM*V | ✔ | ✔ | Only if using the linear regression longitudinal model the mean estimate of the SD of the residual error term in the predicted final visit response based on the response at this visit. |
| TCH Alpha <model> <visit> | LM*V | ✔ | ✔ | Only if using the time course hierarchical longitudinal model the mean estimate of the exponential coefficient of the proportion of the dose response and per-subject response seen at this visit. |
| TCH Lambda <model> <visit> | LM*V | ✔ | ✔ | Only if using the time course hierarchical longitudinal model the mean estimate of the SD of the residual error term in the predicted final visit response based on the response at this visit. |
| TCH Tau <model> | LM | ✔ | ✔ | Only if using the time course hierarchical longitudinal model the mean estimate of the SD of the per subject random effect |
| ITP K <model> | LM | ✔ | ✔ | Only if using the ITP longitudinal model the mean estimate of the ITP shape parameter |
| ITP Lambda <model> | LM | ✔ | ✔ | Only if using the ITP longitudinal model the mean estimate of the SD of the residual error. |
| ITP Tau <model> | LM | ✔ | ✔ | Only if using the ITP longitudinal model the mean estimate of the SD of the per subject random effect |
| ITP Omega <group> <arm> | G*2 | ✔ | ✔ | Only if using the ITP longitudinal model the mean estimate of the mean treatment arm effect. |
| Longmod Resp <Group> <Arm> <Visit> | G*A*V | ✔ | ✔ | Only if using the time course hierarchical or ITP longitudinal model the mean estimate of the response at each visit, reported per group per arm per visit |
Contents of Simulations_freq.csv
| Column Title | Number of columns | Description |
|---|---|---|
| # Trial | 1 | The number of the simulation. |
| Overall Group p-value | 1 | The ANCOVA overall group p-value |
| Overall Trt p-value | 1 | The ANCOVA overall treatment p-value |
| Group x Trt p-value | 1 | The ANCOVA treatment-group interaction p-value |
| Est. Trt Effect <group> | G | The mean estimate of the treatment effect for each group. |
| Lower CI Trt Effect <group> | G | The lower bound of the 95% confidence interval for the estimate of the treatment effect for each group. |
| Upper CI Trt Effect <group> | G | The upper bound of the 95% confidence interval for the estimate of the treatment effect for each group. |
| p-value Trt Effect <group> | G | The p-value for the within group treatment effect for each group. |
| Sigma | 1 | The estimate of the standard deviation in the subjects’ final response. |
| Beta | 1 | The mean estimate of the baseline adjustment |
| Beta SD | 1 | The standard deviation of the estimate of the baseline adjustment |
Contents of PatientsNNNNN.csv
| Column Title | Number of columns | Description |
|---|---|---|
| #Subject | 1 | The subject id number, starting at 1. |
| Region | 1 | Region index |
| DateInWeeks | 1 | The date, in weeks from the start of the trial, of the subject’s baseline visit and randomization. |
| Group | 1 | The index number of the group the subject belongs to. |
| Arm | 1 | A flag indicating the arm the subject was randomized to: 0 = Control, 1 = Study Treatment. |
| LastVisit# | 1 | The index of the last visit for which subject data is available |
| Dropout | 1 | A flag indicating if the subject has dropped out, 0 = not dropped out, 1= dropped out. |
| Baseline | 1 | The baseline. Column is only present if baseline is included. |
| Visit <visit> | V | The subject’s endpoint score for that visit: -9999 = not available. |
Contents of MCMCNNNNN.csv
The MCMC file if requested for output by the user, contains all the MCMC samples for the fitted parameters in the design. There is one row per sample (including the burnin) and the samples from all the analyses in the simulation are included. The first two columns are the analysis index and the sample (within analysis) index. The remaining columns are the parameters whose sample values are being reported, the number and constituents of these columns are highly variable depending on design of the statistical analysis.
| Column Title | Number of columns | Description |
|---|---|---|
| Analysis | 1 | The index of the analysis (interim) in the simulation |
| Sample | 1 | The index of the sample within the analysis |
| TrtResp <group> | D | The estimate of the mean treatment difference in each group |
| Overall Theta | 1 | The overall estimate of the treatment difference |
| CtlResp <group> | The estimate of the mean response on the control arm in each group | |
| Overall Gamma | The overall mean response on the control arms | |
| Sigma | 1 | The estimate of the SD of the endpoint |
| Longmod <model> <param> <visit> these vary significantly from design to design depending on the model used and the number of model instances. The parameter names correspond to the symbols used for the parameters on the Design > Longitudinal tab | L * P * V | If the analysis includes longitudinal modelling then the samples of the parameters of the longitudinal models are output. The number of models depends on the number of different longitudinal fits the user has specified (one over all arms, one for control and one for all treatment arms, model all arms separately, etc.), the number of parameters the model has, and if these are fitted per visit (which depends on the model being fitted), then the number of visits. |